搜索
NEWS
Check category
側邊欄
Time of issue:2020-03-11 00:00:00

Detailed
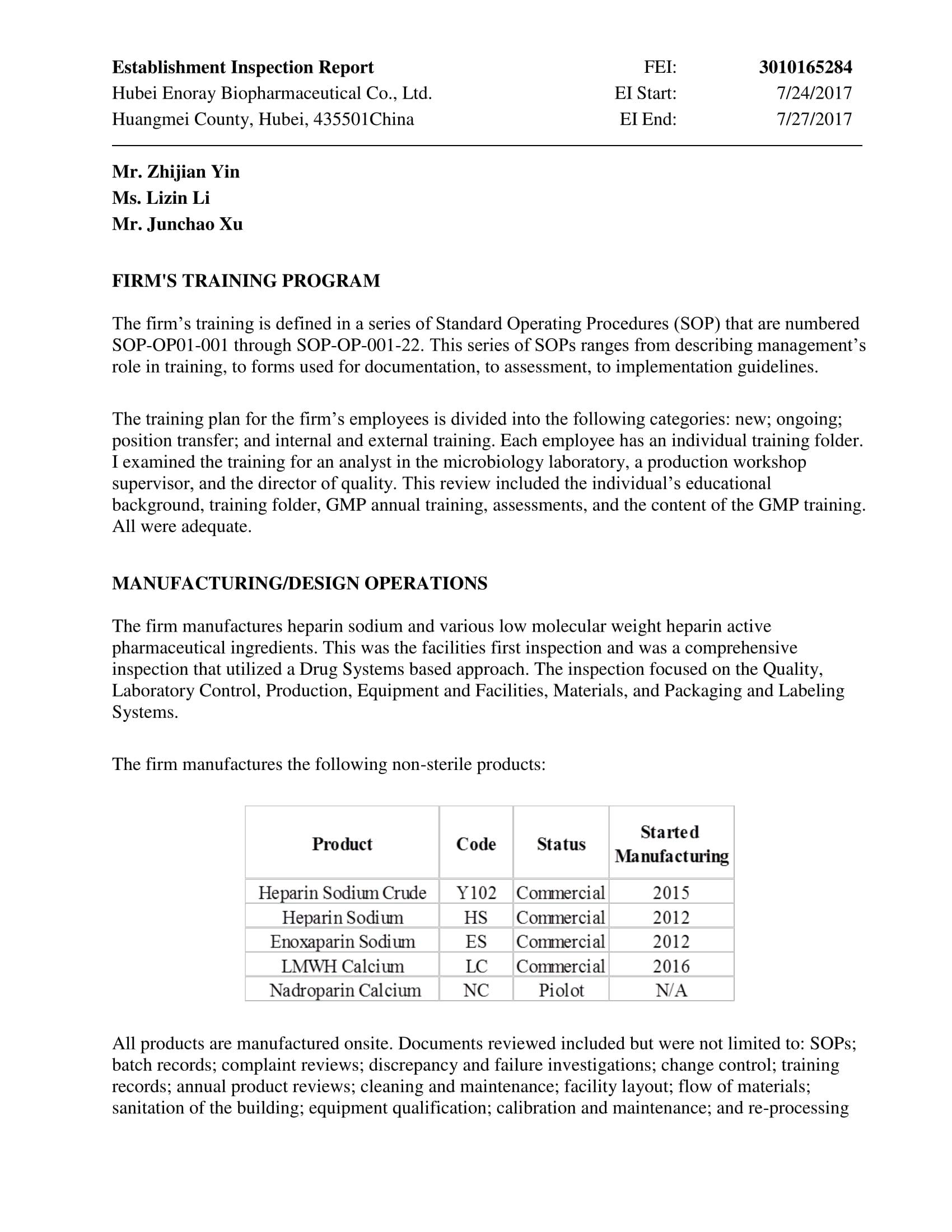
EIR from US FDA
- Categories:Company news
- Author:
- Origin:
- Time of issue:2017-12-13 12:00
- Views:
(Summary description)
EIR from US FDA
(Summary description)
- Categories:Company news
- Author:
- Origin:
- Time of issue:2017-12-13 12:00
- Views:
Information
Congratulations!
We received EIR issued by US FDA.
No Form FDA 483, Inspectional Observations, was issued at the closing of this inspection......
Covering Heparin sodium crude, Heparin sodium, Enoxaparin sodium, LMWH calcium and
Nadroparin calcium.


Scan the QR code to read on your phone
Previous:
CPhI China 2018 from Jun. 20th to 22nd
Next:
CPhI India 2017
Previous:
CPhI China 2018 from Jun. 20th to 22nd
Next:
CPhI India 2017
Copyright Hubei Enoray Biopharmaceutical Co., Ltd ADD:NO.108,Yanjiang Road, Xiaochi Town, Huangmei County, Hubei,435501, China